設施農業:智慧感測【化學系/葉鎮宇特聘教授】
| 論文篇名 | 英文:tert-Butylpyridine Coordination with [Cu(dmp)2]2+/+ Redox Couple and Its Connection to the Stability of the Dye-Sensitized Solar Cell 中文:4-叔丁基吡啶與2,9-二甲基-1,10-菲囉啉銅錯化物支配位化學及其對染敏化太陽能電池穩定性之影響 |
| 期刊名稱 | ACS Applied Material Interfaces |
| 發表年份,卷數,起迄頁數 | 2020, 12 (5): 5812−5819 |
| 作者 | Kannankutty, Kala; Chen, Ching-Chin; Vinh Son Nguyen; Lin, Yu-Cheng; Chou, Hsien-Hsin; Yeh, Chen-Yu(葉鎮宇)*; Wei, Tzu-Chien* |
| DOI | 10.1021/acsami.9b19119 |
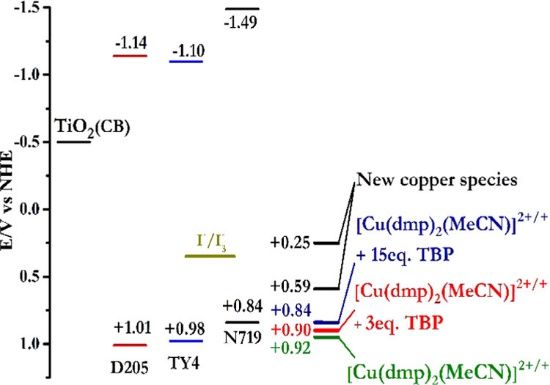
| 中文摘要 | 銅一價/二價氧化還原對因具有低重組能及低氧化還原電位等優點而被應用於具高電壓之染料敏化太陽能電池中,然而近期研究發現做為電解質之銅錯化物容易受到電解液中常見添加物4-叔丁基吡啶之配位而失去原本性質,導致元件之長效性不佳。在此篇論文中,我們選用2,9-二甲基-1,10-菲囉啉銅錯化物做為研究對象,探討4-叔丁基吡啶配位至銅離子之反應機制與對銅離子電化學性質的影響。結果顯示在電解液配方中4-叔丁基吡啶配位至銅離子後會取代2,9-二甲基-1,10-菲囉啉成為配位基,共會形成3種新的銅錯合物。長效性實驗發現元件的填充因子顯著下降,但開路電壓以及短路電流並無明顯改變,是為以銅錯化物為電解質染料敏化太陽能電池元件之穩定性特點。 |
| 英文摘要 | Redox couples play an important role in determining the overall photovoltaic performance of a dye-sensitized solar cell (DSSC). Until the successful implantation of Cu(I)/(II)-complexes in DSSC recently, the innovation on redox couples is slow when compared with leaping and fascinating progress on sensitizers and mesoporous semiconductor films over the last two decades. Cu(I)/(II)-complexes are of particular interest because of their low reorganization energy between Cu(I) and Cu(II) that minimizes potential loss during sensitizer regeneration and allows the open circuit voltage of the device over 1.0 V. However, Cu(I)/(II)-based redox couples are reported to coordinate with 4-tert-butylpyridine (TBP), which is a standard additive in the electrolyte to upshift the Fermi level of TiO2 photoanode, and this is believed to account for the poor durability of a Cu(I)/(II)-based DSSC. Despite of TBP-coordination on Cu(I)/(II)-complexes are confirmed in several reports, its detailed mechanisms have yet to be directly proven. In addition, how TBP coordination with Cu(I)/(II)-complexes affects the long-term stability of the device is never reported. In this study, we choose bis(2,9-dimethyl-1,10-phenanthroline) copper(I)/(II) ([Cu(dmp)22+/+]) as the modeling redox couple to investigate its interaction with TBP. It is found [Cu(dmp)2+] is resistive to TBP coordination but [Cu(dmp)22+] could form three new TBP-coordinated compounds. Although the redox potential of these three new compounds are all negatively shifted when compared to original [Cu(dmp)22+/+], it is confirmed their electrochemical activity on Pt catalyst and mass transfer capability are both demoted significantly after TBP coordination. As a result, serious fill factor (FF) loss is observed on the stability trail while short circuit current density (JSC) and open circuit voltage (VOC) are relatively unaffected. Unlike typical degradation on JSC or VOC of a DSSC, this unique degradation pattern (FF loss) may resemble a feature of Cu(I)/(II)-based redox couple after TBP poison. |