循環農業:農業剩餘資材及生質衍生物高值化轉換再利用【環境工程學系/林坤儀特聘教授】
| 論文篇名 | 英文: Direct growth of nano-worm-like Cu2S on copper mesh as a hierarchical 3D catalyst for Fenton-like degradation of an imidazolium room-temperature ionic liquid in water 中文:奈米蠕蟲狀 Cu2S 在銅網上的直接生長作為分級 3D 催化劑,用於水中咪唑鎓室溫離子液體的芬頓降解 |
| 期刊名稱 | Journal of Colloid and Interface Science |
| 發表年份,卷數,起迄頁數 | 2023, 638, 39-53 |
| 作者 | Jiang, Xin-Yu; Kwon, Eilhann; Wen, Jet-Chau; Bedia, Jorge; Thanh, Bui Xuan; Ghotekar, Suresh; Lee, Jechan*; Tsai, Yu-Chih; Ebrahimi, Afshin; Lin, Kun-Yi Andrew(林坤儀)* |
| DOI | 10.1016/j.jcis.2023.01.029 |
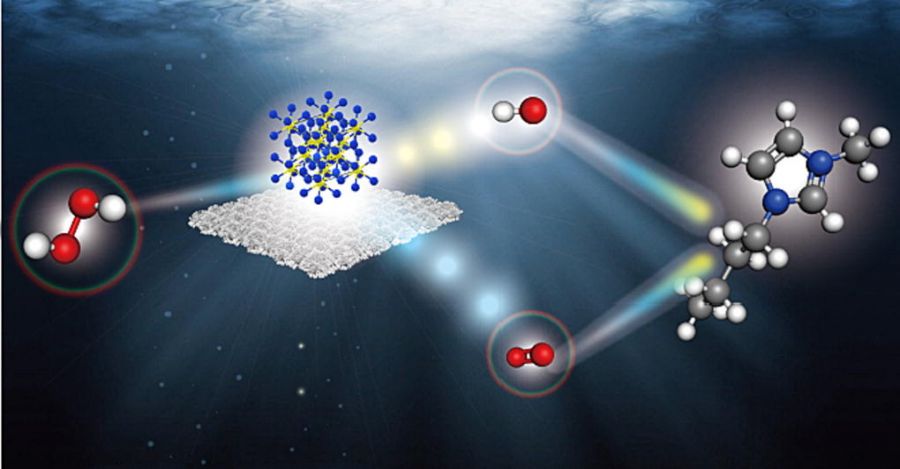
| 中文摘要 | 室溫離子液體(RTIL)消耗量的不斷增加不可避免地將RTIL釋放到水環境中,由於RTIL的毒性對水生生態造成嚴重威脅。 因此,迫切需要開發從水中去除RTIL的有用方法,而最常見的RTIL氯化1-丁基-3-甲基咪唑鎓(C4mimCl)將是研究從水中去除RTIL的最具代表性的RTIL。 由於過氧化氫 (HP) 高級氧化過程被證實是消除新興污染物的有效方法,因此開發有利的多相催化劑來活化 HP 是成功降解 C4mim 的關鍵。 在此,利用CM作為Cu源,透過在銅網(CSCM)上生長Cu2S來製造分層結構。 與其前體CuO@CM相比,此CSCM表現出極高的催化HP有效降解C4mim的催化活性,因為CSCM表現出更優越的電化學性能和反應位點,使得CSCM能夠快速降解C4mim。 CSCM 也表現出比文獻中的所有值更小的 C4mim 消除 Ea。 CSCM 在 NaCl 和海水存在的情況下也表現出活化 HP 降解 C4mim 的高能力和穩定性。 此外,CSCM活化的HP消除C4mim的機制研究也已被闡明,並將其歸因於OH和1O2。 也可以透過量子計算化學詳細檢查和揭示消除途徑,證實CSCM是催化HP降解RTIL的有用催化劑。 |
| 英文摘要 | The increasing consumption of room-temperature ionic liquids (RTILs) inevitably releases RTILs into the water environment, posing serious threats to aquatic ecology due to the toxicities of RTILs. Thus, urgent needs are necessitated for developing useful processes for removing RTILs from water, and 1-butyl-3-methylimidazolium chloride (C4mimCl), the most common RTIL, would be the most representative RTIL for studying the removal of RTILs from water. As advanced oxidation processes with hydrogen peroxide (HP) are validated as useful approaches for eliminating emerging contaminants, developing advantageous heterogeneous catalysts for activating HP is the key to the successful degradation of C4mim. Herein, a hierarchical structure is fabricated by growing Cu2S on copper mesh (CSCM) utilizing CM as a Cu source. Compared to its precursor, CuO@CM, this CSCM exhibited tremendously higher catalytic activity for catalyzing HP to degrade C4mim efficiently because CSCM exhibits much more superior electrochemical properties and reactive sites, allowing CSCM to degrade C4mim rapidly. CSCM also exhibits a smaller Ea of C4mim elimination than all values in the literature. CSCM also shows a high capacity and stability for activating HP to degrade C4mim in the presence of NaCl and seawater. Besides, the mechanistic investigation of C4mim elimination by CSCM-activated HP has also been clarified and ascribed to OH and 1O2. The elimination route could also be examined and disclosed in detail through the quantum computational chemistry, confirming that CSCM is a useful catalyst for catalyzing HP to degrade RTILs. |
| 發表成果與本中心研究主題相關性 | 透過本研究可進一步建立開發本研究計算所需之觸媒材料,並釐清可適合應用之環境條件! |