新穎材料農業:友善環境農業新穎材料研發與安全評估【土壤環境科學系/鄒裕民特聘教授、劉雨庭副教授/優聘教師】
| 論文篇名 | 英文:Inhibitory effects and mechanisms of low-molecular-mass organic acids (LMMOAs) toward Cr(III) oxidation 中文:低分子量有機酸對於三價鉻氧化的抑制作用與機制 |
| 期刊名稱 | Journal of Cleaner Production |
| 發表年份,卷數,起迄頁數 | 2021, 313, 127726 |
| 作者 | Ng, Kim Hoong; Liu, Yu-Ting(劉雨庭); Chang, Chung Tse; Chiang, Po-Neng; Teah, Heng Yi; Chang, Po Hsiang; Tzou, Yu-Min(鄒裕民)* |
| DOI | 10.1016/j.jclepro.2021.127726 |
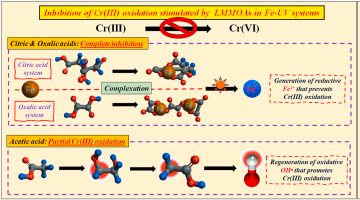
| 中文摘要 | 六價鉻經由光還原成三價鉻的反應已被研究多時,作為減輕六價鉻毒性的方法之一,三價鉻氧化回六價鉻也扮演著同等重要的角色,但相關研究卻付之闕如。因此,本研究將探討低分子量有機酸包括檸檬酸、草酸、醋酸,在三價鐵及紫外光存在的環境下對三價鉻氧化的抑制作用與機制。研究結果顯示最顯著的三價鉻氧化(300 μM 三價鉻中的~4 μM)發生在 pH 3.0、添加 400 μM 三價鐵及照射 12 小時紫外光的系統中;然而這樣程度的三價鉻氧化卻可完全或部分被低分子量有機酸抑制。檸檬酸與草酸幾乎可完全抑制三價鉻氧化,即便是最低濃度(50 μM)的添加,這是基於二價鐵的生成可以結合氫氧自由基。至於醋酸,50 μM 的添加量僅可達到 75%的抑制率,其甚至降低到 50%當添加量增加到 400 μM。與檸檬酸與草酸不同的是,醋酸不傾向與三價鐵結合,當多餘的醋酸與氫氧自由基反應時會產生更多的氫氧自由基,加上醋酸系統中二價鐵的生成量比檸檬酸與草酸系統中少了 28-35%。在有過多氫氧自由基與不足量的二價鐵的狀況下,多餘的氫氧自由基便會誘發三價鉻氧化,導致較弱的氧化抑制作用。本研究證明檸檬酸、草酸、醋酸在三價鐵存在的系統中,對於三價鉻的氧化都有抑制作用,提供了一個可行且環境友善的方法,避免三價鉻氧化,造成二次汙染。 |
| 英文摘要 | Photo-reduction of Cr(VI) into Cr(III) has been extensively studied for the alleviation of Cr(VI) hazards. However, knowledge in regard to the Cr(III) oxidation back into Cr(VI), which serves an equally important role in controlling Cr pollution, is limited. To this end, inhibitory effects and related mechanisms of low-molecular-mass organic acids (LMMOAs), including citric acid, oxalic acid and acetic acid, toward Cr(III) oxidation in the presence of Fe(III) under UV-irradiation were determined in this study. Results showed that the most prominent Cr(III) oxidation (~4 μM out of 300 μM Cr(III)) occurred at pH 3.0 in the presence of 400 μM Fe(III) and 12 h of UV-irradiation, yet such Cr(III) oxidation could be entirely or partially suppressed upon the addition of LMMOAs. Both citric and oxalic acids triggered a nearly complete inhibition of Cr(III) oxidation, even with the lowest dosage of 50 μM, ascribed to promoted generation of Fe(II) that scavenged OH• radicals. Regarding acetic acid, the addition of 50 μM only yielded 75% inhibitory efficiency, and further decreased to 50% as its amount increased to 400 μM. In contrast with citric/oxalic acids, acetic acid tended not to complex with Fe(III). While such free acetic acid reacted with OH• radicals generated from photolysis of Fe(OH)2 , additional OH• radicals were produced. Furthermore, the Fe(II) generation in the acetic acid system was about 28–35% of that in the citric/oxalic acid systems. In the case of supplementary OH• radicals and insufficient Fe(II), less OH• radicals could be consumed, and thereby the amendment of acetic acid showed a relatively weak inhibitory effect toward Cr(III) oxidation. This study evidenced that all three LMMOAs are effective toward the inhibition of Cr(III) oxidation in the presence of Fe(III). This feasible, practical, and environmental friendly approach is promising to prevent the secondary pollution caused by Cr(III) oxidation. |
| 發表成果與本中心研究主題相關性 | 使用環境中常見的有機酸與三價鐵來避免六價鉻的二次汙染,確保土壤與水資源健康,達到環境永續。 |